OSHA compliance for med spas in 2025 is non-negotiable. If your business offers treatments like Botox, microneedling, or laser procedures, you must meet federal safety standards to protect employees and patients. Failure to comply can lead to fines, legal issues, or even business closures.
Here’s what you need to know:
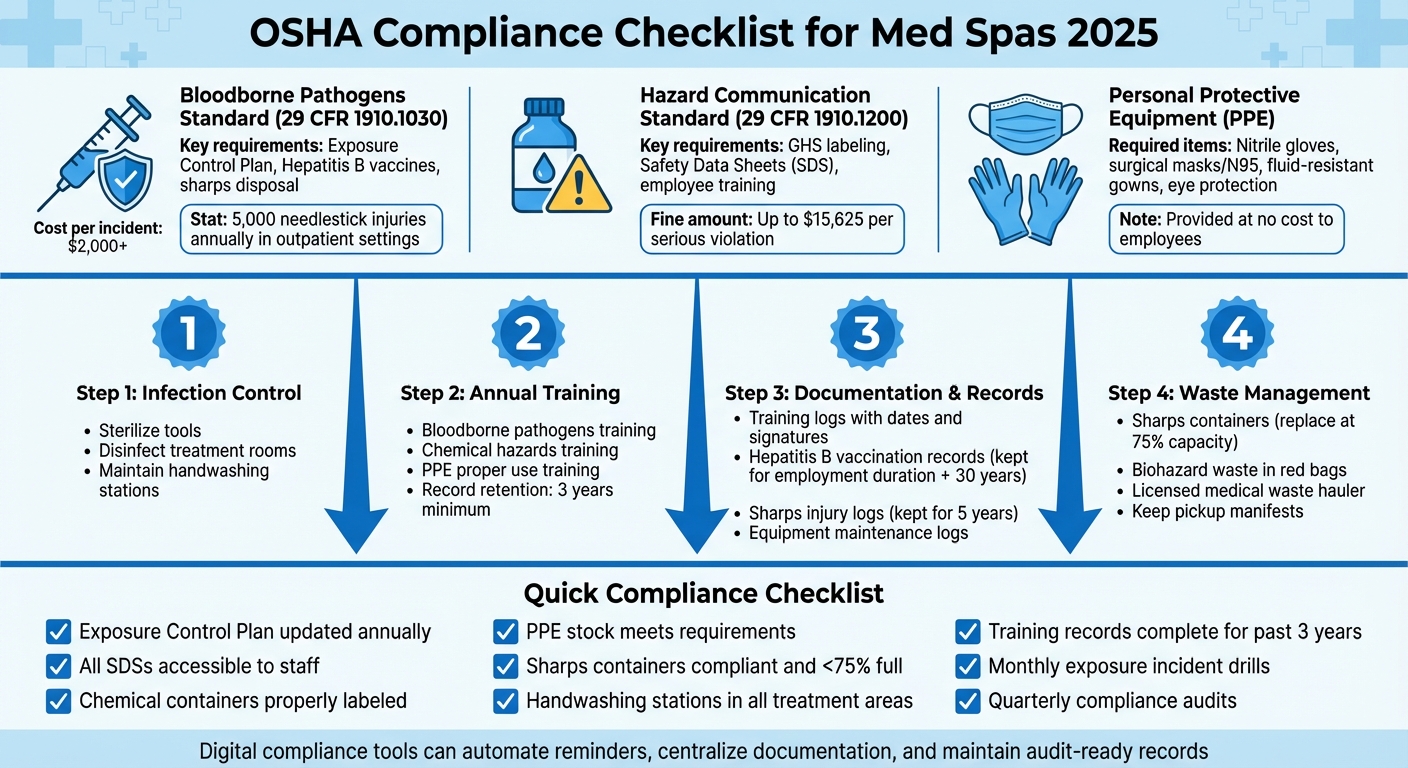
- Bloodborne Pathogens Standard (29 CFR 1910.1030): Requires an Exposure Control Plan, Hepatitis B vaccines for employees, and proper sharps disposal.
- Hazard Communication Standard (29 CFR 1910.1200): Mandates chemical labeling, Safety Data Sheets (SDS), and regular employee training.
- Personal Protective Equipment (PPE): Employers must provide gloves, masks, gowns, and eye protection at no cost, with training on proper use.
Key steps for compliance:
- Maintain strict infection control protocols, including sterilizing tools and disinfecting rooms.
- Provide annual training on bloodborne pathogens and chemical hazards.
- Keep detailed records of training, waste disposal, and equipment maintenance.
- Ensure sharps containers and biohazard waste meet OSHA standards.
Using digital tools like Prospyr can simplify managing training, documentation, and compliance tasks. Staying organized helps avoid penalties and ensures a safe environment for staff and clients.
OSHA Compliance Checklist for Med Spas 2025
2025 OSHA Standards That Apply to Med Spas
Med spas must adhere to several OSHA standards designed to address the specific risks associated with aesthetic treatments. These regulations cover everything from safe handling of needles during injectables to proper storage of chemicals used in peels and disinfectants. Knowing which standards apply to your facility is essential for creating a safe and compliant workplace. Below, we break down the key regulations and what they mean for med spa operations.
Bloodborne Pathogens Standard (29 CFR 1910.1030)
This regulation is aimed at protecting employees from exposure to blood and other potentially infectious materials (OPIM) during procedures like Botox injections, dermal fillers, microneedling, PRP therapy, IV treatments, and laser procedures that involve skin penetration. Med spas must develop an Exposure Control Plan that identifies risks, outlines engineering controls, and specifies post-exposure protocols such as immediate medical evaluation and incident reporting.
Facilities are also required to:
- Offer Hepatitis B vaccinations at no cost to employees within 10 working days of their initial assignment if they face occupational exposure.
- Use puncture-resistant sharps containers, maintain handwashing stations, and follow documented sterilization procedures.
- Provide annual training to employees.
OSHA estimates that outpatient settings experience roughly 5,000 needlestick injuries each year, with each incident costing over $2,000. These measures are part of broader infection control practices discussed later in this guide.
Hazard Communication Standard (29 CFR 1910.1200)
Med spas handle various hazardous chemicals, including TCA peels, disinfectants, sterilants, and anesthetic agents, all of which fall under this standard. Facilities must implement a written hazard communication program and ensure that:
- All chemical containers are labeled according to GHS guidelines, including signal words, pictograms, and hazard statements.
- Safety Data Sheets (SDSs) are accessible for all hazardous substances.
Common violations, such as missing SDS binders or unlabeled disinfectant bottles, can lead to fines of up to $15,625 per serious infraction. Proper chemical storage practices, such as keeping incompatible substances (e.g., acids and bases) separate, are critical. Employees must receive both initial and annual training on topics like chemical inventory management, SDS interpretation, and emergency procedures. Training records should be kept for three years, and chemical inventory lists should be updated quarterly.
Personal Protective Equipment (PPE) Requirements
Med spas must conduct hazard assessments for each procedure and treatment room to determine the appropriate personal protective equipment (PPE) needed. Employers are required to provide PPE at no cost to employees, which may include:
- Nitrile gloves for handling blood or chemicals.
- Surgical masks or N95 respirators for aerosol-generating treatments like lasers.
- Fluid-resistant gowns to protect against splashes.
- Eye protection for procedures such as chemical peels or cryotherapy.
Training on proper PPE use - including how to put it on, take it off, and perform respirator fit tests - must be documented. Facilities should also enforce regular replacement schedules and conduct compliance audits every six months to ensure adherence. Creating procedure-specific PPE checklists (e.g., gloves and goggles for laser treatments or full gowns for microneedling) and stocking PPE stations in treatment rooms can further enhance safety. Failure to comply with PPE requirements can result in significant fines.
Proper PPE use is a cornerstone of workplace safety and ties into the broader safety systems discussed throughout this guide.
Creating a Safe and Sanitary Med Spa Environment
Meeting OSHA's infection control standards involves more than just keeping things tidy. Med spas must implement rigorous sanitation measures to manage risks associated with bloodborne pathogens, chemicals, and clinical waste. This includes establishing clear protocols for sterilizing equipment, maintaining treatment spaces to healthcare-grade standards, and keeping detailed records to safeguard both clients and staff.
To ensure thorough cleaning, establish written schedules that specify tasks, timing, and responsibilities. Between clients, treatment beds and non-porous surfaces should be disinfected using EPA-approved healthcare disinfectants, following the manufacturer's recommended contact times. High-touch areas like door handles, counters, and payment devices need daily attention. Weekly tasks should include deep cleaning treatment rooms, inspecting ventilation systems, and checking the expiration dates of disinfectants. These detailed cleaning routines are the foundation for effective infection control, which will be further explored in later sections.
For instruments used in procedures like injections or microneedling that involve contact with blood or potentially infectious materials, strict guidelines apply. Items must either be single-use or sterilized according to their classification. Autoclaves and other sterilizers should undergo regular testing with biological indicators, and test results must be logged to confirm their effectiveness. Single-use items like needles and microneedling tips should never be reused. Instead, they must be discarded immediately into puncture-resistant sharps containers.
Infection Control and Sanitation Protocols
Adhering to OSHA's standards requires med spas to enforce strict infection control practices. Hand hygiene is a cornerstone of these efforts. Staff should wash their hands with soap and water or use alcohol-based hand rubs before and after each patient interaction, after removing gloves, and after touching potentially contaminated surfaces. Handwashing stations equipped with soap and disposable towels should be available in all treatment areas, and alcohol-based hand rubs (with at least 60% alcohol) should be strategically placed. However, hand rubs are not a substitute for washing when hands are visibly dirty.
Personal protective equipment (PPE) is another critical component. Staff must use proper techniques for putting on and removing PPE and dispose of contaminated items correctly to avoid cross-contamination.
Treatment room turnover should follow procedure-specific protocols. For instance, after a laser treatment that might involve bleeding, all equipment and surfaces must be disinfected, contaminated materials disposed of, and fresh linens prepared. Service-specific checklists can help standardize these processes.
Sharps Disposal and Medical Waste Management
OSHA's approach to sharps safety begins with engineering controls. Use safety-engineered needles when possible and place puncture-resistant sharps containers within arm's reach of the treatment area. These containers must meet FDA requirements: they should be rigid, leak-proof, closable, and clearly labeled or color-coded (typically red with a biohazard symbol). Position them at eye level and replace them before they are three-quarters full to minimize the risk of needle-stick injuries.
Sharps should be disposed of immediately after use, without recapping or passing them hand-to-hand. Common compliance issues in med spas include improper sharps and waste disposal, missing written exposure control plans, and inadequate training on hazardous materials - any of which can lead to infections, staff injuries, fines, or even forced closures.
Proper waste management is equally important. Items like blood-soaked materials, certain tubing, and other regulated waste must be placed in clearly labeled biohazard or red bags. These should be stored in a secure area, away from public spaces and clean supplies, until a licensed medical waste hauler collects them. Keep manifests and pickup records as proof of compliance, as inspectors often request these documents. Additionally, state regulations may impose stricter requirements than federal OSHA rules, so be sure to account for local guidelines.
A HIPAA-compliant platform like Prospyr can simplify managing these protocols. It centralizes training documentation, task tracking, and compliance records. Digital tools can assign and timestamp cleaning duties, store staff training attestations, and help managers monitor overdue tasks or equipment maintenance. Communication tools allow quick updates when protocols change, and analytics can highlight trends - like increased sharps usage - that may require additional waste pickups or safety assessments.
Employee Training and Documentation Requirements
After reviewing OSHA standards and safe practices, it’s clear that structured training and thorough documentation are essential for compliance. OSHA mandates that med spas provide training to all employees at the time of hire, annually, and whenever new exposure risks arise. For employees handling needles, blood, or other potentially infectious materials, completing Bloodborne Pathogens Standard training is a must. Similarly, staff who work with chemicals - whether injectables or disinfectants - must receive instruction under the Hazard Communication Standard. This training should include reviewing Safety Data Sheets (SDS), proper labeling, and safe handling procedures. Other key areas to cover include the correct use of personal protective equipment (PPE), sharps safety, incident reporting, and emergency response procedures tailored to the specific services offered.
Employers are responsible for ensuring that employees not only complete training but also demonstrate competency. Tools like checklists can help verify skills such as proper PPE use, sterile field setup, and sharps disposal. Supervisors should document these evaluations, and additional measures like short quizzes or hands-on demonstrations can confirm knowledge retention. If gaps in understanding are identified, targeted retraining should follow, with all remediation efforts documented.
Staff Training and Exposure Control Plans
For med spas with potential exposure to blood or other infectious materials, having a written Exposure Control Plan (ECP) is non-negotiable. This plan should outline tasks and job roles that involve exposure - such as injections, microneedling, IV therapy, or PRP treatments - and describe the measures in place to minimize risks. The ECP must specify engineering controls (like safety-engineered sharps), work practices (e.g., avoiding needle recapping), PPE requirements, and steps to take after an exposure incident. It should also cover procedures for housekeeping, decontamination, offering Hepatitis B vaccinations, post-exposure evaluations, and scheduled training. Importantly, the ECP must be reviewed and updated at least annually or whenever new procedures, devices, or technologies are introduced.
Training can be delivered through a combination of in-person sessions, vendor-led device safety demonstrations, and online OSHA modules. Using role-based training matrices ensures that employees receive instruction tailored to their specific job risks. Periodic drills, such as simulated needlestick incidents, and brief "toolbox talks" can reinforce key practices between formal training sessions.
Recordkeeping and Documentation Standards
Proper recordkeeping is a cornerstone of OSHA compliance. Essential documentation for med spas includes the ECP, hazard communication program, PPE policies, and infection control protocols. Employers must maintain detailed training logs, including dates, topics covered, trainer credentials, and attendee signatures. Additional records should document Hepatitis B vaccination offers or declinations, post-exposure evaluations, and needlestick incidents. Employers should also ensure that SDSs are easily accessible - whether in binders or electronically - alongside records of equipment maintenance, routine housekeeping, and safety inspections.
Retention requirements vary depending on the type of record. Exposure and medical records must be kept for the duration of employment plus 30 years, while training logs should be retained for at least three years. Sharps injury or incident logs need to be kept for a minimum of five years after the calendar year in which the incident occurred. When exposure incidents happen, such as a needlestick or blood splash, it’s crucial to document the event thoroughly. This includes noting the circumstances, the device involved, the task being performed, and any contributing factors. The ECP should guide the immediate response, and employers must coordinate post-exposure evaluations, source patient testing (if allowed), employee testing and counseling, and any recommended prophylaxis. Corrective actions, such as adopting safer devices or revising protocols, should also be documented.
Using a HIPAA-compliant platform like Prospyr can simplify record management. These systems allow administrators to attach training completion certificates, set reminders for renewals, and link training records directly to individual employees. Digital dashboards can integrate OSHA policies, ECPs, SDSs, and checklists with staff profiles, ensuring training and competency records remain up to date. This streamlined approach not only keeps administrators organized but also makes it easy to generate reports on completed training, safety meetings, and written plans - minimizing disruptions during OSHA inspections. These documentation practices work hand-in-hand with the broader safety measures outlined earlier.
sbb-itb-02f5876
OSHA Compliance Checklist for Med Spas
This checklist highlights key areas med spas need to address to meet OSHA standards. It’s a practical tool for owners and managers to spot and fix compliance gaps. Start by ensuring your Exposure Control Plan is up-to-date - reviewed within the last year - and includes clear procedures for handling blood exposure incidents. Also, confirm that Safety Data Sheets (SDSs) for all chemicals, like disinfectants and topical anesthetics, are readily available to staff, whether in physical binders or through electronic systems. Don’t forget to check that all secondary chemical containers are properly labeled.
Take a walk through your facility to review Personal Protective Equipment (PPE) availability and condition. Make sure your PPE stock meets OSHA requirements and that sharps containers are compliant - these should never exceed 75% capacity. Additionally, ensure your medical waste hauler provides proper documentation for pickups. Check that each treatment area has accessible handwashing stations equipped with soap, paper towels, and, as a backup, alcohol-based hand sanitizer.
Next, dive into documentation. Look at training records from the past three years to confirm annual bloodborne pathogens and hazard communication training sessions were conducted. These records should include sign-in sheets and quiz results with at least an 80% pass rate. Verify that every employee’s Hepatitis B vaccination status is documented and that your sharps injury log is complete for the past five years. This log should include details of needlestick injuries, post-exposure evaluations, and corrective actions. For example, failure to comply with these requirements led to significant fines during a 2023 incident in California.
Once your documentation is in order, focus on routine drills and audits to maintain compliance. Schedule monthly mock drills to practice handling exposure incidents. Conduct quarterly audits of cleaning logs, equipment maintenance records, and biohazard waste documentation. Digital tools can simplify this process. Platforms like Prospyr, which are HIPAA-compliant, can automate training reminders, maintain digital logs for PPE inspections and sanitation protocols, and timestamp records using AI-generated notes. These systems help minimize manual errors and keep everything audit-ready in one centralized location. By making compliance part of your regular routine, you’ll create a safer environment for both your staff and your patients.
How Practice Management Software Supports OSHA Compliance
Navigating OSHA compliance can be a daunting task, especially when relying on outdated methods like paper binders, spreadsheets, or scattered sign-in sheets. These manual systems often lead to outdated documents, missed training deadlines, and added stress during inspections. Practice management software simplifies this process, turning compliance from a reactive chore into a proactive, streamlined system.
Take Prospyr's HIPAA-compliant platform as an example. It consolidates critical documents like your Exposure Control Plan, Safety Data Sheets, hazard communication policies, and training materials into a single, secure, and searchable library. With version control in place, you can rest assured that everyone is accessing the most up-to-date protocols. Staff members can even electronically confirm they've reviewed policy updates, keeping you audit-ready at all times. Plus, the platform sends alerts for key deadlines, such as annual bloodborne pathogens training, Hepatitis B vaccination offers, and policy reviews, ensuring nothing slips through the cracks.
Daily infection control and sanitation tasks are also made easier with digital checklists that integrate directly into treatment protocols. For instance, a room can’t be marked “ready” until staff log essential steps like disinfection, sharps container checks, and equipment sterilization. Each action is timestamped and linked to the specific user, creating a detailed, auditable record that aligns with OSHA’s recordkeeping standards.
In cases of incidents like needlesticks or chemical exposures, standardized templates capture essential details - such as date, time, circumstances, PPE used, and follow-up actions. These templates also track steps like post-exposure evaluations and corrective actions, ensuring nothing is overlooked. Administrators can generate reports to monitor training completion rates, overdue modules, sanitation task records, and incident trends across multiple locations. This compliance dashboard provides a clear view of potential gaps, allowing you to address issues before they escalate into violations.
Conclusion
Following OSHA guidelines goes beyond just steering clear of penalties - it lays the groundwork for a safer workplace, shields your clients, and helps reduce potential legal and financial troubles. Implementing bloodborne pathogen protocols, proper sharps disposal, and requiring personal protective equipment are critical steps in preventing needlestick injuries, lowering infection risks, and cutting down on workers' compensation claims. These measures also help avoid post-procedure complications that could damage your reputation or result in malpractice issues. Prioritizing OSHA compliance ensures the safety of your team, the well-being of your clients, and the strength of your professional reputation.
FAQs
What OSHA regulations should med spas follow in 2025 to ensure safety and compliance?
In 2025, med spas will need to comply with several OSHA regulations to ensure a safe and compliant workplace. This includes the correct handling and storage of hazardous materials, implementing rigorous infection control protocols, and providing thorough safety training for staff. Additionally, med spas must follow OSHA's guidelines concerning bloodborne pathogens, chemical safety, and the proper use of equipment.
By keeping current with these regulations, med spas can safeguard both their employees and clients, creating a secure and professional environment.
What’s the best way for med spas to handle OSHA documentation and staff training?
Med spas can streamline OSHA documentation and staff training with an all-in-one practice management platform like Prospyr. This platform offers tools to track training sessions, manage digital forms, and securely store important records, making it easier to meet OSHA standards while cutting down on administrative work.
By consolidating these tasks in one place, med spas can stay organized, keep records accurate, and dedicate more time to providing top-notch patient care.
What happens if a med spa doesn’t follow OSHA regulations?
Failing to comply with OSHA regulations can have serious repercussions for med spas. These can range from hefty fines and legal battles to the possibility of the business being shut down entirely. Non-compliance also raises the likelihood of workplace injuries, putting both employees and patients at risk.
But the consequences don’t stop there. Neglecting OSHA standards can tarnish your med spa’s reputation, leading to a loss of patient trust - a critical factor for maintaining long-term success. Following these regulations isn’t just about avoiding penalties; it’s about creating a safe and professional space for everyone, from staff to clients.



