When selling aesthetic products in the U.S., proper labeling is not just a suggestion - it’s a legal requirement. Non-compliance can lead to product seizures, recalls, and damaged reputation. Here's what you need to know:
- Key Laws: Labels must comply with the Federal Food, Drug, and Cosmetic (FD&C) Act, the Fair Packaging and Labeling (FP&L) Act, and the Modernization of Cosmetics Regulation Act (MoCRA) of 2022.
- Principal Display Panel (PDP): Ensure product identity and net quantity are clear, proportionate, and visible.
- Information Panel: Include accurate manufacturer details and a complete ingredient list in descending order by weight.
- Warnings: Highlight safety risks with bold, legible text on contrasting backgrounds.
- MoCRA Updates: As of January 6, 2026, over 14,000 facilities and nearly 1 million products are registered with the FDA. New rules require allergen disclosure and adverse event reporting.
Staying compliant protects your business and builds consumer trust. Regular audits and technology tools, like the FDA’s Cosmetics Direct platform, can simplify this process.
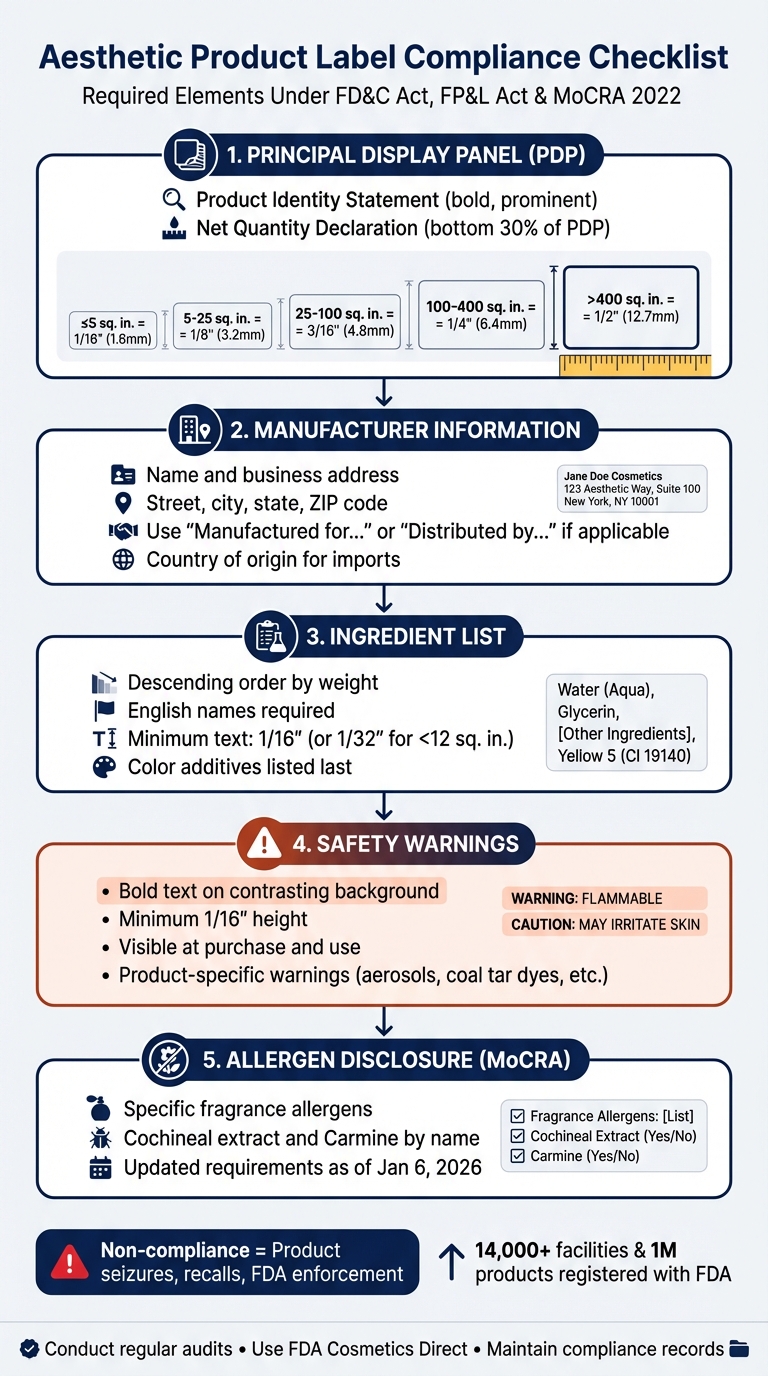
5-Step Aesthetic Product Label Compliance Checklist for U.S. Regulations
Principal Display Panel (PDP) Requirements
The Principal Display Panel (PDP) is the part of your label that's most visible to customers when your product is on display. If your packaging can be viewed from multiple angles, you’ll need to repeat all required information on each visible panel. For rectangular packages, the PDP is defined as one full side. For cylindrical containers, it’s calculated as 40% of the height multiplied by the circumference. It’s also essential to make sure your product identity clearly conveys what the product is.
Product Identity Statement
The product identity is a critical aspect of the PDP, as it’s often the first thing customers notice. It should be displayed prominently in bold on the PDP. Use a descriptive, common, or even fanciful name that gives a clear sense of what the product is. Including an illustration that shows the product’s intended use can also help clarify its identity.
The text size should be proportional to the most prominent printed elements on the PDP, and all text should be aligned parallel to the base of the package when it’s displayed. This makes the product identity easy to read at a glance.
Net Quantity of Contents
The net quantity declaration must be placed within the bottom 30% of the PDP. It should be separated from other text by a space equal to the letter height and twice the width of the letter "N". For packages with a PDP area of 5 square inches or less, this placement rule is waived.
Use U.S. customary units for the declaration. Liquid products should be measured in fluid ounces (fl. oz.) at 68°F, while solid or semi-solid products should use weight in ounces (oz.). For items weighing between 1 to 4 pounds or containing between 1 pint to 1 gallon, include a dual declaration, such as "24 fl. oz. (1 pt 8 fl. oz.)". Metric equivalents can be added, but they cannot replace the required U.S. units.
| PDP Area | Minimum Type Height |
|---|---|
| 5 sq. in. or less | 1/16 inch (1.6 mm) |
| More than 5 to 25 sq. in. | 1/8 inch (3.2 mm) |
| More than 25 to 100 sq. in. | 3/16 inch (4.8 mm) |
| More than 100 to 400 sq. in. | 1/4 inch (6.4 mm) |
| Over 400 sq. in. | 1/2 inch (12.7 mm) |
Avoid using exaggerated terms like "Giant Pint" or "Economy Size" on the PDP. These can lead to misbranding violations. Stick to standard abbreviations such as "wt." for weight, "fl. oz." for fluid ounce, and "lb." for pound.
Meeting these PDP guidelines is essential for complying with U.S. labeling standards. Precise adherence to these rules ensures your label remains compliant and clear to consumers.
Information Panel Requirements
The information panel plays a key role in ensuring your product meets compliance standards. Unlike the Principal Display Panel (PDP), the information panel is any other area on your packaging where consumers are likely to look for critical details at the time of purchase. This is where you should include the manufacturer or distributor information and the complete ingredient list. Keep in mind, placing required information on the bottom of the package is discouraged, unless the packaging is very small.
Manufacturer or Distributor Information
Your label must include the name and full business address of the manufacturer, packer, or distributor. This address should contain the street address, city, state, and ZIP code. However, the street address can be omitted if your business is listed in a current city or telephone directory.
"The name and business address appearing on the label may be those of the manufacturer, packer or distributor." – FDA Cosmetics Labeling Guide
Make sure to use your official corporate name. If the name and address on the label don’t belong to the actual manufacturer, include a qualifying statement such as "Manufactured for..." or "Distributed by..." before the name. For imported items, you must also indicate the English name of the country of origin, as required by the Tariff Act of 1930.
Ingredient List Formatting
Ingredients should be listed in descending order by weight, using their common English names. The FDA does not accept Latin or foreign terms (e.g., "Aqua" or "Parfum") as substitutes for English names, though these can appear in parentheses after the English term (e.g., "Water (Aqua)"). Ingredients that make up 1% or less of the product, as well as color additives, can be grouped together and listed in any order after the ingredients present in higher concentrations.
The text for ingredient declarations must be legible, with letters no smaller than 1/16 inch (or 1/32 inch for surfaces smaller than 12 square inches). For fragrances and flavors, you can simply use the terms "fragrance" or "flavor" without detailing every component. If your product serves a dual purpose (both drug and cosmetic), list the drug ingredients first as "active ingredients," followed by the cosmetic ingredients labeled as "inactive ingredients" in descending order of weight.
Additional Labeling Requirements
Beyond the basic display and information panel standards, these added elements ensure labels meet federal regulations while prioritizing consumer safety.
Required Safety Warnings
Safety warnings are essential to prevent health risks. According to 21 CFR 740.1, these warnings must be bold, placed on a contrasting background, and located where they are easily visible at the time of purchase and use.
"The label of a cosmetic product shall bear a warning statement whenever necessary or appropriate to prevent a health hazard that may be associated with the product." - 21 CFR 740.1
The text for warnings must be at least 1/16 inch tall. Specific product types also have unique warning requirements. For instance, aerosol products must include the following:
"Warning - Avoid spraying in eyes. Contents under pressure. Do not puncture or incinerate. Do not store at temperature above 120°F. Keep out of reach of children."
Similarly, coal tar hair dyes must provide detailed patch-test instructions, while bubble bath products need warnings about potential urinary tract irritation in children.
If a product's safety has not been confirmed by qualified experts, the Principal Display Panel must include: "Warning - The safety of this product has not been determined."
Allergen Disclosure
All ingredients must be listed on the information panel in descending order by weight. Traditionally, fragrance and flavor components have been labeled generically as "fragrance" or "flavor." However, under the Modernization of Cosmetics Regulation Act (MoCRA), manufacturers must now disclose specific fragrance allergens. Certain substances, such as Cochineal extract and Carmine, must be identified by their precise names. It's crucial to reference the latest MoCRA allergen list to ensure compliance. Addressing allergen transparency not only meets legal standards but also improves label clarity.
Label Formatting and Text Size
All mandatory label information must be easy to read and understand under normal purchasing conditions. Text should contrast clearly with the background and remain unobstructed by designs or graphics. The minimum letter height for ingredient lists is 1/16 inch, but for packages with a total surface area under 12 square inches, it can be reduced to 1/32 inch. Warning statements, however, must always maintain a minimum height of 1/16 inch.
Letter size is measured by the height of the lowercase "o" when mixed-case lettering is used. For net quantity declarations, the ratio of letter height to width cannot exceed 3:1. Additionally, all required text must appear in English. If another language is included, the same information must also be provided in that language.
sbb-itb-02f5876
How to Maintain Label Compliance
Staying compliant with labeling rules is an ongoing process. With regulations constantly evolving and products frequently updated, businesses need to conduct regular reviews and implement strong systems to ensure compliance.
Regular Compliance Audits
Conduct periodic reviews of every label to confirm that the five key elements - Identity, Net Quantity, Business Information, Ingredient Declaration, and Warning Statements - are included and accurate. Regularly cross-check ingredient lists against the FDA's database of prohibited and restricted ingredients.
Make sure ingredient lists follow the INCI naming conventions and are arranged in descending order of predominance. For ingredients used at 1% or less, they can appear in any order after the main ingredients, but color additives must always be listed last. Use digital tools or manual methods to verify that font sizes meet the minimum requirements for the Principal Display Panel (PDP) area.
All product claims should be accurate and avoid suggesting disease treatment, as such claims could result in the product being classified as a drug. To comply with MoCRA, ensure labels are updated to include new allergen disclosures and professional use information when necessary.
"It is illegal to introduce a misbranded cosmetic into interstate commerce, and such products are subject to regulatory action".
Incorporating digital tools can make these audit processes more efficient and less prone to error.
Using Technology for Compliance Management
Technology has made compliance management more accessible and efficient. For example, the FDA's Cosmetics Direct platform simplifies facility registration and product listing under MoCRA.
For businesses managing multiple product lines, platforms like Prospyr offer centralized compliance tracking. These tools maintain a "golden PDF" version of labels, preventing unauthorized changes and ensuring consistency. Automated systems also improve traceability by linking batch and lot codes to digital records, making audits, recalls, and documentation much easier to manage.
Conclusion
Staying compliant with labeling regulations is an essential part of protecting both your business and your customers. While the Federal Food, Drug, and Cosmetic Act and the Fair Packaging and Labeling Act set the legal groundwork, it's important to note that the FDA does not pre-approve cosmetic labels. This means the responsibility for meeting all regulatory requirements falls squarely on the manufacturer or distributor.
Non-compliance can lead to serious consequences. A product is deemed "misbranded" if its label is false, misleading, lacks required information, or if the necessary details aren’t displayed clearly. The FDA has the authority to take strong actions against misbranded products, including product seizures, injunctions, and even criminal charges. Past enforcement cases serve as a stark reminder of the risks businesses face when they fail to meet labeling standards.
"It is the manufacturer's and/or distributor's responsibility to ensure that products are labeled properly. Failure to comply with labeling requirements may result in a misbranded product."
- U.S. Food and Drug Administration
The Modernization of Cosmetics Regulation Act of 2022 (MoCRA) has brought the most significant updates to FDA oversight of cosmetics since 1938. With new requirements like mandatory facility registration, product listing, and safety substantiation, compliance is no longer a one-time task. Instead, it requires ongoing vigilance. Tools such as the FDA’s Cosmetics Direct portal and services like Prospyr help businesses, including multi-product clinics, streamline compliance efforts.
In a beauty market expected to grow nearly 5% annually through 2030, staying compliant isn’t just about avoiding penalties - it’s about maintaining your competitive edge. Regular label reviews, accurate record-keeping, and adapting to regulatory changes are critical steps in safeguarding your brand and ensuring success in this expanding industry. Consistent compliance not only minimizes risks but also reinforces your position in the market.
FAQs
What laws regulate aesthetic product labeling in the United States?
In the United States, labeling for aesthetic products is governed by the Federal Food, Drug, and Cosmetic Act (FD&C Act) and the Fair Packaging and Labeling Act (FPLA). These regulations are in place to ensure that product labels deliver information that is accurate, truthful, and not misleading.
Some of the key rules include disclosing ingredients clearly, ensuring product claims are accurate, and identifying the manufacturer or distributor on the label. Following these guidelines helps uphold transparency and fosters trust among consumers.
What changes does the Modernization of Cosmetics Regulation Act (MoCRA) bring to product labeling?
The Modernization of Cosmetics Regulation Act (MoCRA) brings tougher rules for cosmetic product labels, emphasizing clarity, accuracy, and consumer protection. Under these new guidelines, labels must now clearly disclose details such as potential allergens, usage limitations (like "for professional use only"), and instructions on how to report any adverse reactions.
The goal of these updates is to empower consumers with the knowledge they need to make informed choices while ensuring brands remain accountable to updated safety and labeling standards. For companies, staying compliant isn't just about following the law - it's about preserving trust and avoiding potential regulatory complications.
How can businesses ensure their product labels stay compliant with regulations?
Keeping up with FDA requirements for cosmetic labeling is crucial for businesses. Regularly reviewing product labels ensures they include all the necessary details, such as product identity, net contents, ingredient lists, and contact information for the responsible business. Since regulations and industry standards can change over time, staying updated is key.
To avoid mistakes and maintain consistency, businesses can adopt internal processes like:
- Using version control to track label changes
- Creating detailed checklists for compliance
- Providing staff with proper training on regulatory standards
Conducting regular label audits is especially important when launching new products or making claims. These audits can help identify and address potential issues before they become problems. Additionally, working with regulatory experts or legal advisors on a periodic basis can provide valuable guidance, ensuring products remain compliant while also fostering consumer trust.